Xeh4 Lewis Structure. Perform a symmetry analysis to determine the symmetries of the molecular orbitals formed from linear combinations of the atomic. In the brcl3 lewis structure bromine br is the least electronegative atom and goes in the center of the lewis structure.
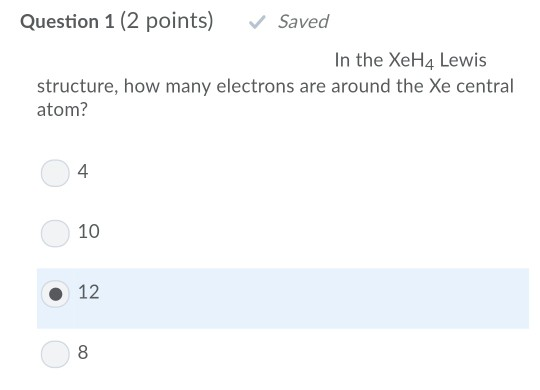
Furthermore what is the lewis structure for brcl3. For the xe atom valence orbitals are arranged from lowest to highest energy in the following order. You ll want to calculate the formal charges on each atom to make sure you have the best lewis structure for xeh 4.
Hydrogen h only needs two valence electrons to have a full outer shell.
You ll want to calculate the formal charges on each atom to make sure you have the best lewis structure for xeh 4. Energy of 5s energy of 5p energy of 4d. Hydrogen h only needs two valence electrons to have a full outer shell. In the brcl3 lewis structure bromine br is the least electronegative atom and goes in the center of the lewis structure.
