Sio2 Lewis Structure Polarity. So in essence sulfur dioxide is polar while carbon dioxide is nonpolar because the individual movements of the bonds in carbon dioxide cancel one another out yet in the case of sulfur dioxide the angular nature of the molecule means that there is an imbalance between the poles that it has both a negative and positive side and therefore the molecule is polar. The system is very polar and attracts hydrogen bonding to the oxygens in the network.
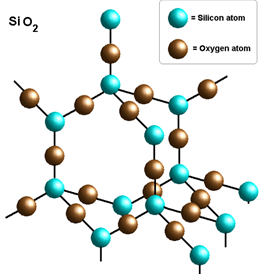
Is so2 polar or non polar. The system is very polar and attracts hydrogen bonding to the oxygens in the network. The molecular geometry of so2 has a bent shape which means the top has less electronegativity and the bottom placed atoms of oxygen have more of it.
Benzene is an organic compound with the molecular formula c6h6.
Lewis dot structure and polarity. How the molecule might react with other molecules. The key is to understand the steps and practice. In this sciencestruck post we provide you with the polarity and steps to create the lewis dot diagram of this aromatic compound.
