Sf4 Lewis Structure Bond Angle. The structure of sf4 can therefore be anticipated using the principles of vsepr theory. It s like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle.
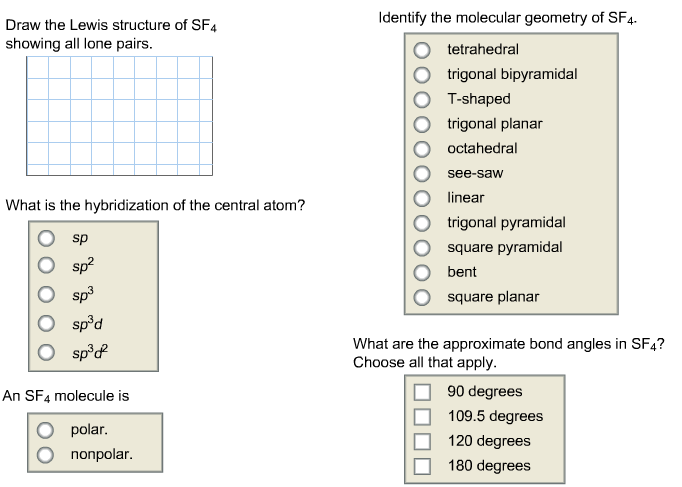
Electron pairs bonding has fewer repulsion when compared to the electrons lone pair. The nature of the molecule is polar. Sf4 sulfur tetrafluoride sulfur tetrafluoride has 5 regions of electron density around the central sulfur atom 4 bonds and one lone pair.
I also go over formal charge hybridization shape and bond angle.
Sf4 molecular geometry is see saw with one pair of valence electrons. These atoms form a trigonal bipyramidal shape. Consequently the molecule has two distinct types of f ligands two axial and two equatorial. The relevant bond distances are s fax 164 3 pm and s feq 154 2 pm.
