Sf2 Lewis Structure Lone Pairs. A spb sp2c sp3d sp3de sp3d2an sf2 molecule isidentify the molecular geometry of sf2. Identify the molecular geometry of sf2.
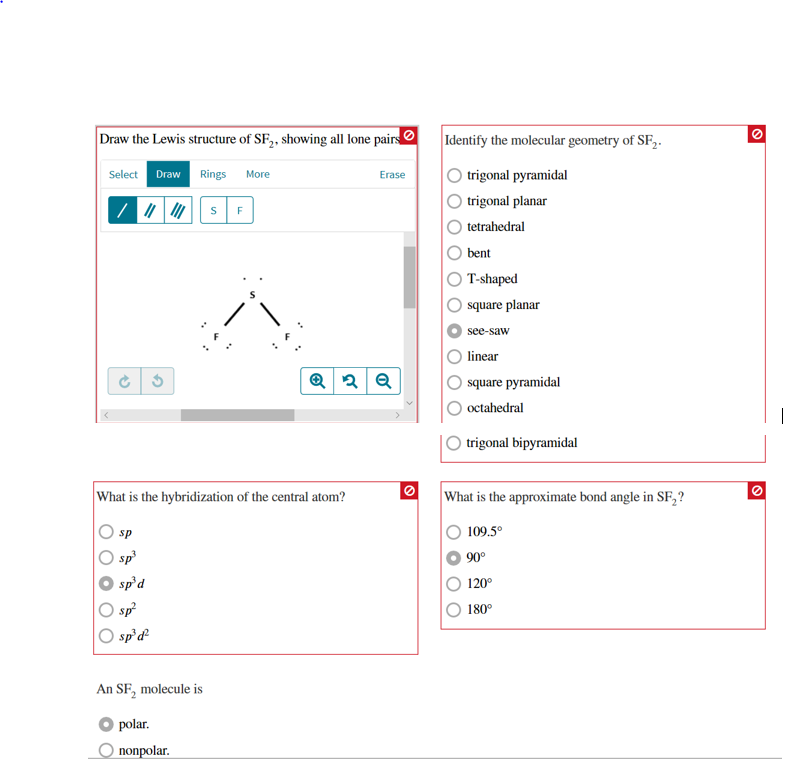
It forms one bond because it has seven valence electrons and it only needs one more to get to eight. Draw the lewis structure of sf2 showing all lone pairs. Twenty minus sixteen so what it tells us is that there are four electrons or two lone pairs of the central sulfur atom and fluorine.
The lewis structure for sf2 is a lot like water where oxygen also has 6 valence electrons and h only makes one bond like f.
The s has two bonds to each of the two f s and that leaves two lone. The s has two bonds to each of the two f s and that leaves two lone. Draw the lewis structure of sf2 showing all lone pairs. The lewis structure for sf2 is a lot like water where oxygen also has 6 valence electrons and h only makes one bond like f.
