Reaction Energy Diagram With Elementary Steps. An elementary reaction is an individual reaction step in a reaction mechanism. In the above reaction a reactant goes through one elementary step with a lower activation energy transition state 1 to form the intermediate.
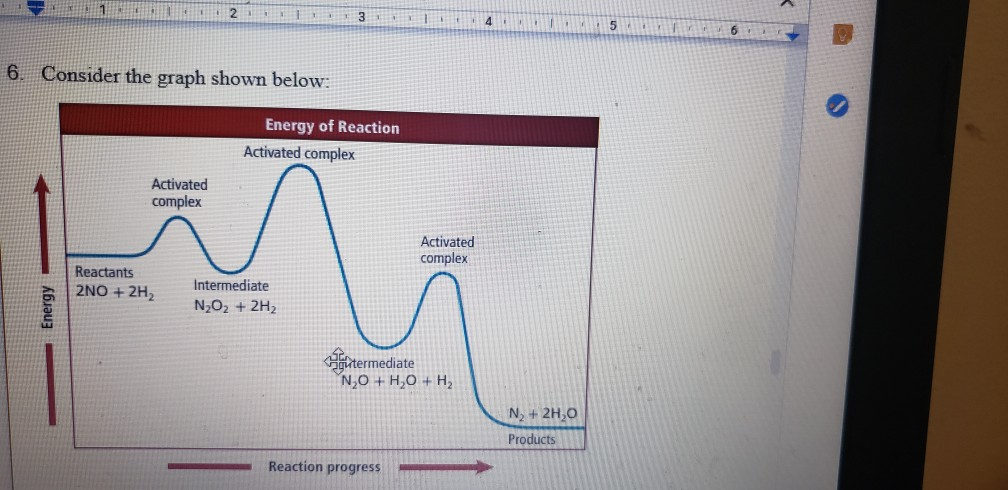
The potential energy diagram shows an activation energy peak for each of the elementary steps of the reaction. The potential energy diagram shows an activation energy peak for each of the elementary steps of the reaction. In this video i go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph.
How many elementary steps are in the reaction mechanism.
I which then reacts further to form the products p. An elementary reaction is an individual reaction step in a reaction mechanism. Pictured below is the energy diagram for a two step reaction. One describes an elementary.
