Oxygen Lewis Dot Structure Ion. The electrons in the outer most energy level of an atom or ion. The lewis structure for oxygen o2 shows the bond between two oxygen atoms.
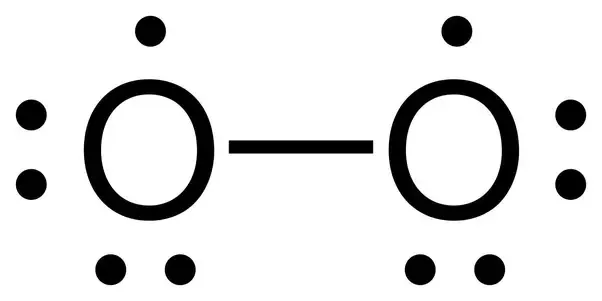
Oxygen gets 6 dots. Now take this number and place a dot for each valence electron. The electrons in the outer most energy level of an atom or ion.
For example oxygen has 6 valence electrons so we write the symbol o for oxygen and surround it with 6 dots.
Lewis structure of co carbon monoxide a carbon monoxide molecule consists of one carbon atom and one oxygen atom. The covalent bond in an oxygen molecule o 2 oxygen gas is non polar electrons are shared equally. For example oxygen has 6 valence electrons so we write the symbol o for oxygen and surround it with 6 dots. Since there are only two oxygen atoms in an o 2 molecule the atoms form a double bond resulting in the following lewis electron dot structure.
