O2 Molecular Orbital Diagram Bond Order. Since a molecule of oxygen has two unpaired. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular.
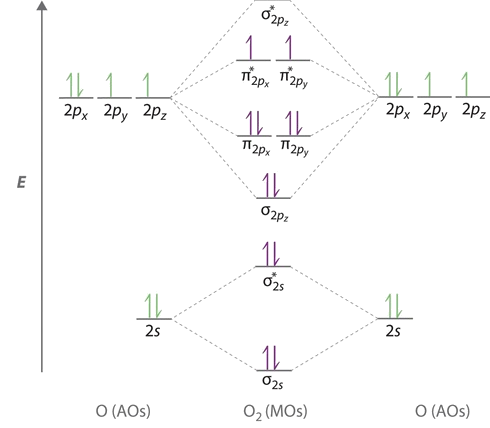
Electronic structure of oxygen atom is leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule represented as kk the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown i electronic configuration ii bond order. Since a molecule of oxygen has two unpaired. The problem provides you with the mo diagram for the c 2 molecule so all you really have to do here is add an electron to that diagram.
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of.
Therefore it is paramagnetic o 2 ion this ion is. Here nb 8. Therefore it is paramagnetic o 2 ion this ion is. The bonding electrons are in the σ2s σ2p π2px and π2py mos giving 2 2 2 2 8.
