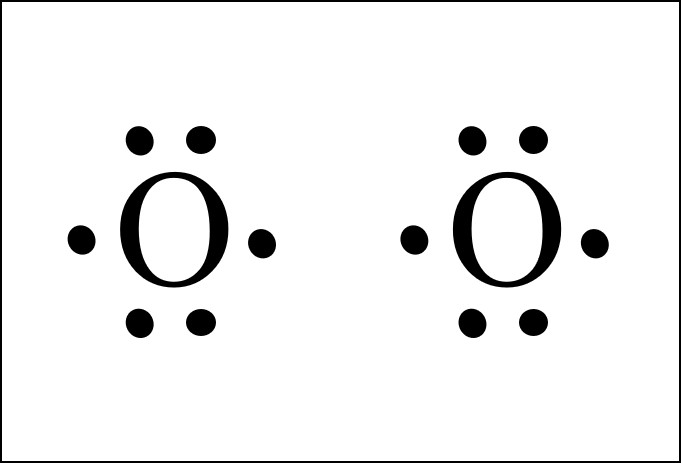
O2 Lewis Structure Valence Electrons. A step by step explanation of how to draw the o2 2 lewis structure peroxide ion for the o2 2 lewis structure calculate the total number of valence elec. So each o is surrounded by 8 total valence electrons giving it an octet and making it stable.
The lewis theory predicts that the formula for a compound of magnesium and sulfur is mgs2. All the valence electrons in the molecule must be accounted for. The rule is especially applicable to carbon nitrogen oxygen and the halogens but also to metals such as sodium or magnesium.
The two letter o s in the o 2 lewis structure represent the nuclei centers of the oxygen atoms.
Valence electrons are the electrons found in the outermost shell of the atom which is referred to as the valence shell. Total valence electrons pairs. Having eight valence electrons is very stable and is called an octet. The lewis structure of oxygen should have 8 valence electrons.
