Nf3 Lewis Structure Molecular Geometry. Easy way lewis structure of nf 3. Although the bond angle should be 109 5 degrees for trigonal pyramidal molecular geometry it decreases to 107 degrees due to the lone pair on the nitrogen atom.
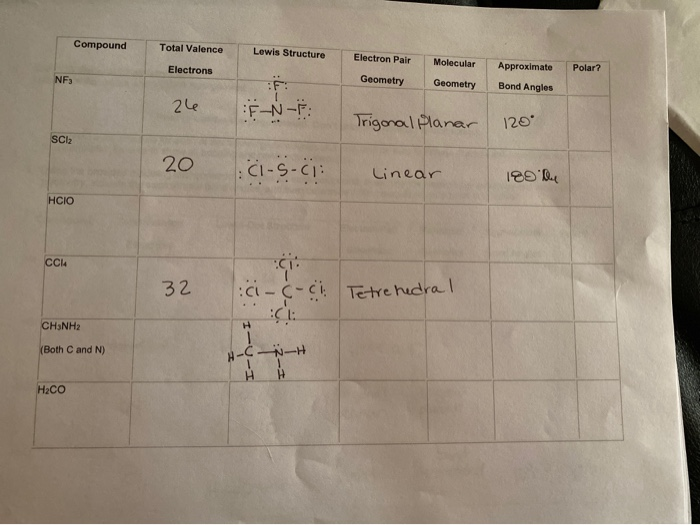
It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. Draw a lewis structure for each of the following molecules. Calculate the total valence electrons in the molecule.
Additionally is bf3 trigonal pyramidal.
Draw a lewis structure for each of the following molecules. This pair exerts repulsive forces on the bonding pairs of electrons. Diagram in pictures database nf3 lewis diagram just. The nf3 is polar because they distribute bonding electrons and its shape also balances the polarity.
