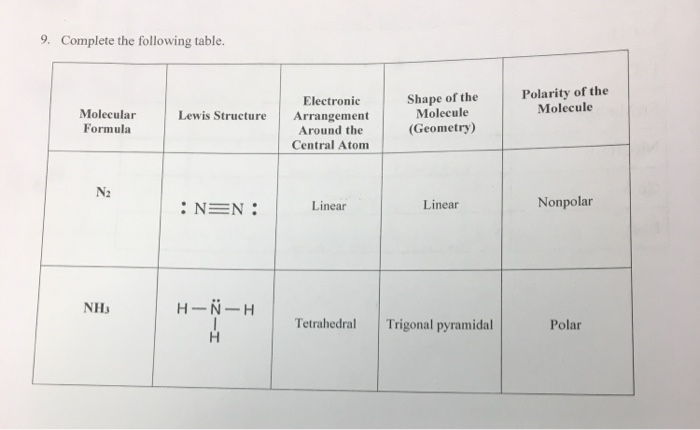
N2 Lewis Structure Polarity. The n 2 lewis structure shows two nitrogen atoms bonded in the same way to each other. Small nonpolar substances tend to be gasses.
Ch 2 o f. However molecular structure is actually three dimensional and it is important to be able to describe molecular bonds in terms of their distances angles and relative arrangements in space a bond angle is the angle between any two bonds that include a common atom usually measured in degrees. Learn to determine if n2 is polar or nonpolar based on the lewis structure and the molecular geometry shape we start with the lewis structure and then use.
In this example we can draw two lewis structures that are energetically equivalent to each other that is they have the same types of bonds and the same types of formal charges on all of the structures both structures 2 and 3 must be used to represent the molecule s structure the actual molecule is an average of structures 2 and 3 which are called resonance structures.
You would end up with n three lines to indicate three bonds n it d look like n n but with one more line in between and each n would have two dots on the end noble gases benzene methane ethylene carbon tetrachloride. Lewis structures shapes and polarity w 319 everett community college student support services program draw lewis structures name shapes and indicate polar or non polar for the following molecules. Learn to determine if n2 is polar or nonpolar based on the lewis structure and the molecular geometry shape we start with the lewis structure and then use. Therefore n 2 is a nonpolar substance.
