Molecular Orbital Diagram Of O2 2 Negative. The electronic configuration of c 2 is kk σ 2s 2 σ 2s 2 π 2p x 2 π 2p y 2. The double bond in c 2 consist of both pi bonds because the four electrons are present in the two pi molecular orbitals.
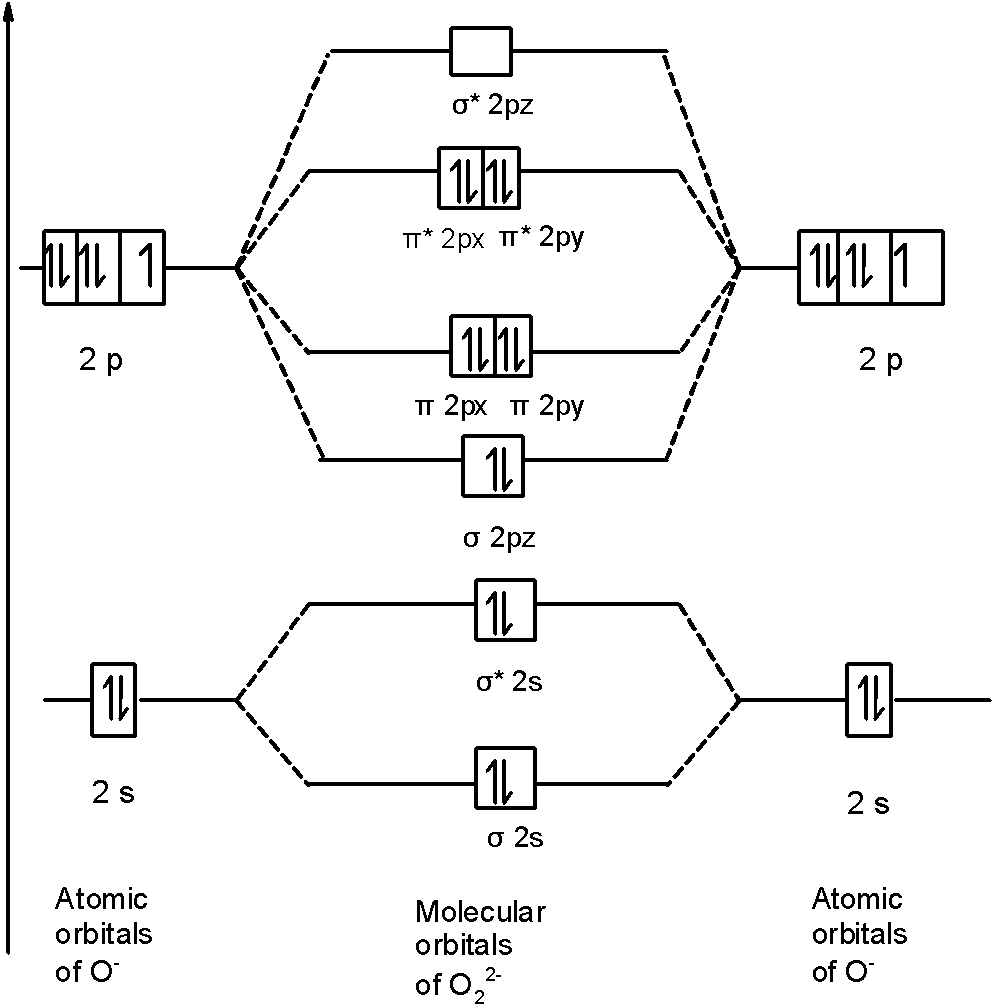
Bo 1 2 bonding e anti bonding e 1 2 8 4 2 lcao mo theory also predicts correctly that o2has two unpaired electrons. Superoxides are compounds in which the oxidation number of oxygen is 1 2. Molecular electron configuration for o2 σ2σ 2σ2π4π 2 we can also calculate the o o bond order.
We illustrate how to use these points by constructing a molecular orbital energy level diagram for f 2 we use the diagram in part a in figure pageindex 1.
Electronic configuration of oxygen molecule bond order in oxygen molecule o 2 number of bonding electrons number of anti bonding electrons 10 6 2 o 2. Experiments show that each o2 molecule has two unpaired electrons. But i don t get how the diagram s supposed to work. The electronic configuration of c 2 is kk σ 2s 2 σ 2s 2 π 2p x 2 π 2p y 2.
