Lithium And Bromine Lewis Dot Structure. Fluorine chlorine and bromine have 7 valence electrons. Oxygen 6.
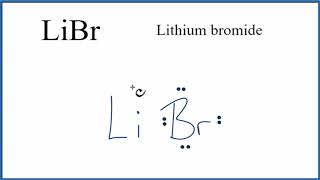
In chemistry the lewis dot structure is a simplified representation of the valence electrons in an atom or a molecule. In order to draw a lewis dot structure you need the element symbol and the number of valence electrons. Each valence electron is represented.
A step by step explanation of how to draw the babr2 lewis dot structure for babr2 we have an ionic compound and we need to take that into account when we dra.
On the other hand bromine is a non metal which has 35 electrons in its single atom. Lithium is an alkali metal which has 3 electrons. Hydrogen 1. Fluorine chlorine and bromine have 7 valence electrons.
