Lewis Structure For Xef2cl2. And same in case of cl atoms. Draw 3 d representations of the two known isomers.
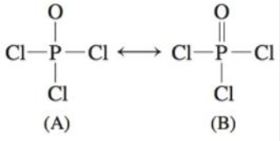
The trial structure has 32 valence electrons. While there are several possible isomeric structure for xef2cl2 only two are known. Write lewis structures for these two compounds and describe how measurement of dipole moments might be used to distinguish between them.
Write lewis structures for these two compounds and describe how the measurement of.
Two different compounds have the formula xef 2 cl 2. What is the geometry around the xe atom. The trial structure has 32 valence electrons. Start with a trial structure.
