Lewis Diagram For F2. F 2 is a reddish gas at room temperature. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist.
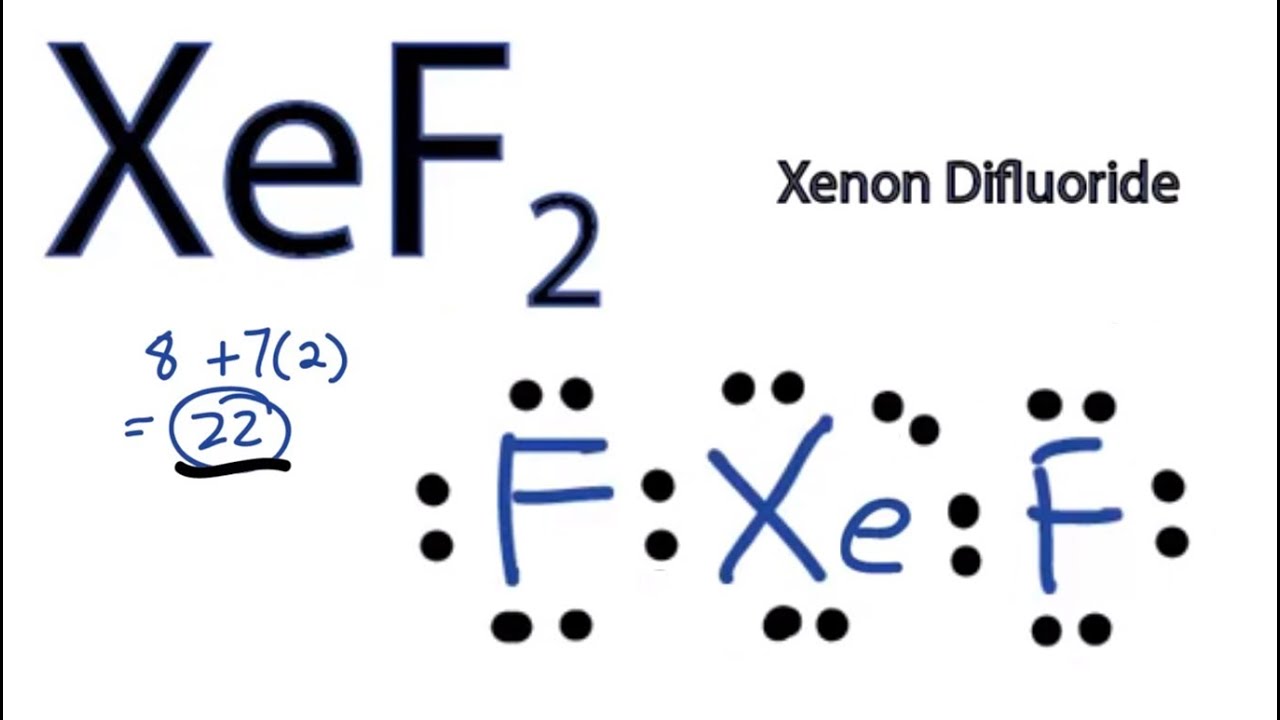
In lewis dot structures each dot in the lewis structures listed below m and x represent various elements in the third period of the periodic table. The second phase of drawing the lewis diagram is determining how many electrons and a given atom requires to be happy or satisfied. And over here.
Drawing the lewis structure for f 2.
70 more lewis dot structures. That fluorine has 8 as well. Learn to determine if f2 is polar or nonpolar based on the lewis structure and the molecular geometry shape we start with the lewis structure and then use. I quickly take you through how to draw the lewis structure of f2 difluorine.
