Hcn Lewis Structure Shape. So we re still using ten valence electrons for the hcn lewis structure but nitrogen has an octet with eight valence electrons carbon has eight valence electrons hydrogen has only two but that s all it needs for a full outer shell. Step method to draw lewis structure of hydrogen cyanide.
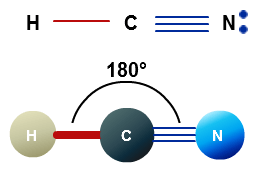
H c n in this example hcn the lewis diagram shows carbon at the center with no lone electron pairs. So that s the lewis structure for hcn. H c n lewis structure of hcn.
The carbon and nitrogen are bonded through.
So that s the lewis structure for hcn. The carbon and nitrogen are bonded through. So we re still using ten valence electrons for the hcn lewis structure but nitrogen has an octet with eight valence electrons carbon has eight valence electrons hydrogen has only two but that s all it needs for a full outer shell. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule.
