Hcl Lewis Structure Polar Or Nonpolar. Hcl is written wrong and probably a typographical error. Thus one of the atoms in the lewis structure for no cannot have an octet.
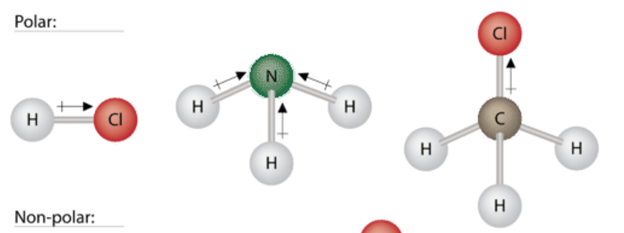
In hcl the hydrogen atom is partially positively charged while the chlorine atom is partially negatively charged. Hope that explains that for you. The shape of the pcl3 is trigonal pyramidal and pcl3 will reacts readily with many other compounds because phosphorous trichloride is a strong oxidizer.
N has 5 valence electrons and o has 6 giving a total of 11.
Hydrogen chloride hcl is a chemical compound of the two elements chlorine and hydrogen. Is not as high as elements like water ammonia etc. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from h atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom. N has 5 valence electrons and o has 6 giving a total of 11.
