Formal Charge Sulfur Dioxide Lewis Structure. In so2 the sulfur s valence electron 6. There are 2 oxygen atoms in the compound thus 6 2 12.
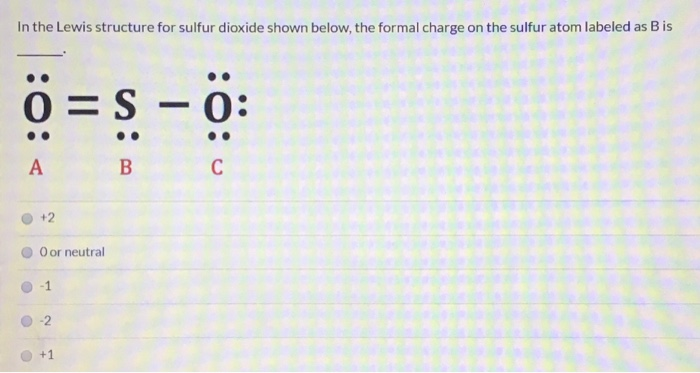
N s f with the nitrogen with four free electrons the sulfur with two and the fluorine with six. Continuing with sulfur we observe that in a the sulfur atom shares one bonding pair and has three lone pairs and has a total of six valence electrons. No2 1 lewis structure.
Nov 16 2012 hi there.
In b the sulfur atom has a formal charge of 0. In c the sulfur atom has a formal charge of 1. N s f with the nitrogen with four free electrons the sulfur with two and the fluorine with six. Step 6 at last it s important to check if all the atoms are having their lowest possible formal charge.
