F2 Lewis Structure Lone Pairs. With core pairs on. Use information from step 4 and 5 to draw the ch 2 f 2 lewis structure.
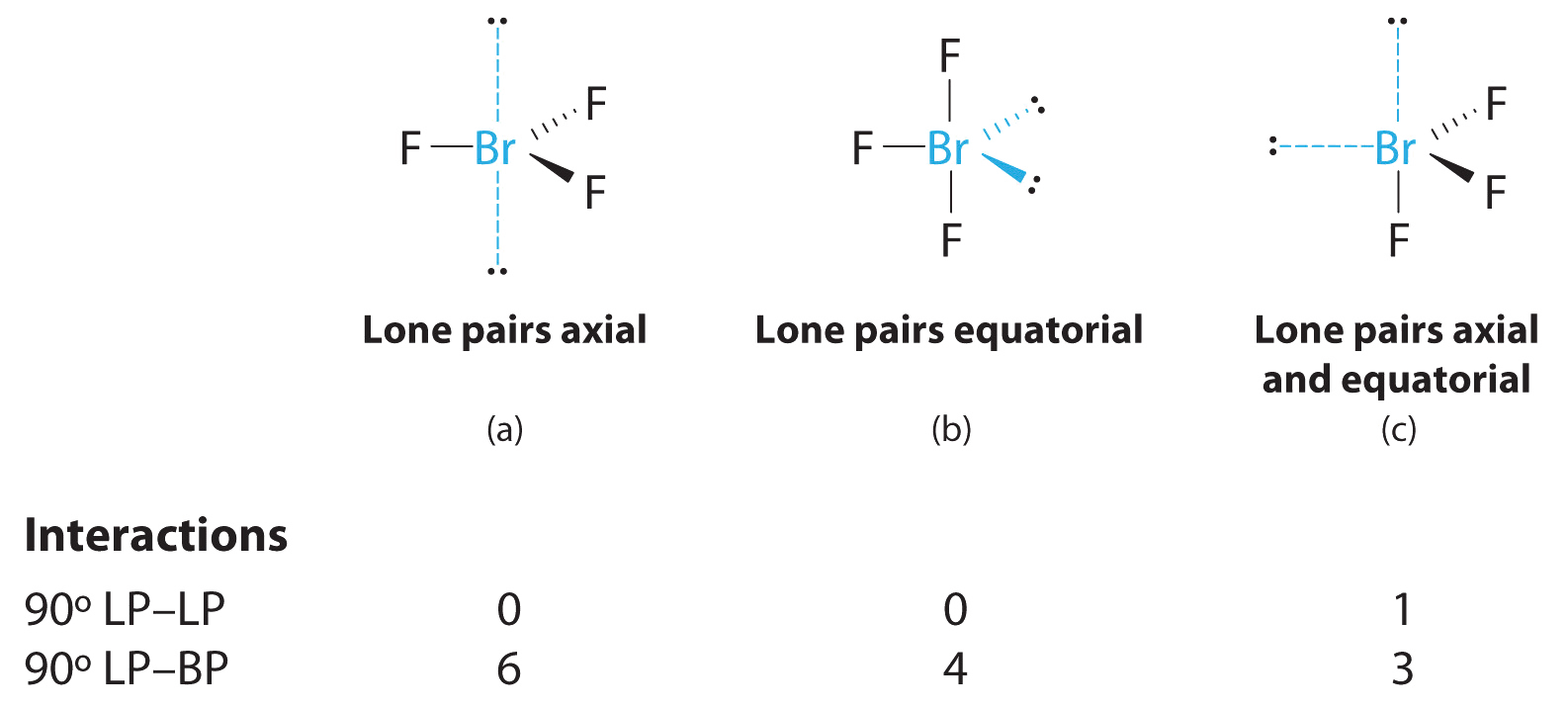
20 8 12e 6 lone pairs. Each f atom also has three pairs of electrons that are not shared with the other atom. A lone pair is a pair of electrons in a lewis electron dot structure that is not shared between atoms.
C 1 f 2 o 3 f 4 top of page.
Finally put the bond pairs and lone pairs of electrons on the atoms. Donor acceptor interactions in the best lewis structure the localized orbitals in your best lewis structure can interact strongly. A lone pair is a pair of electrons in a lewis electron dot structure that is not shared between atoms. C 1 f 2 o 3 f 4 top of page.
