Electron Dot Structure Of S8. The atomic number z of sulphur is sixteen and its electronic configuration is 2 8 6. Exam prep package at.
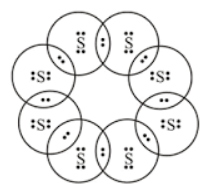
Each sulphur atom is linked to similar atoms on either side by single covalent bonds and thus completes its octet. Lewis who described them in a 1916 article titled the atom and the molecule lewis structures depict the bonds between atoms of a molecule as well as any unbonded electron pairs. The sulphur atom has six valence electrons.
The atomic number z of sulphur is sixteen and its electronic configuration is 2 8 6.
The atomic number z of sulphur is sixteen and its electronic configuration is 2 8 6. Draw its electron dot structure. Lewis structures also known as electron dot structures are named after gilbert n. Draw the electron dot structures for i ethanoic acid ii co2 iii f2 iv ch4.
