Correct Lewis Dot Structure For H2o. There are also two pairs of electrons around the oxygen which you can see at the lewis structure. In order to determine the molecular geometry for h2o observe the lewis structure of the same.
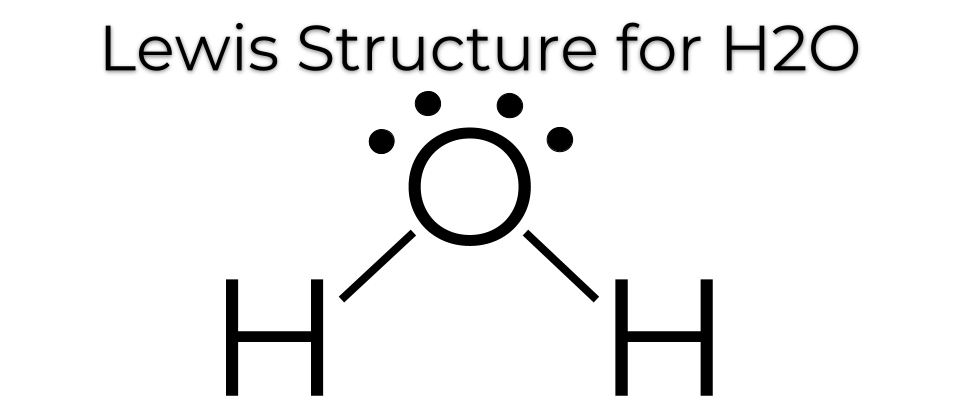
The lewis dot structure of water begins with a single o atom in the center. 2 plus 6 equals 8. On the periodic table hydrogen s in group 1 it has 1 valence electron.
A step by step explanation of how to draw the h2o lewis dot structure water for the h2o structure use the periodic table to find the total number of valenc.
On the right and left sides are a singly bonded h atom. The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. Let s do the lewis structure for water. This bent molecular structure gives it many unique properties such as being polar.
