Cif3 Lewis Dot Structure. 28 6 22. Bear in mind this is not a normal configuration for chlorine.
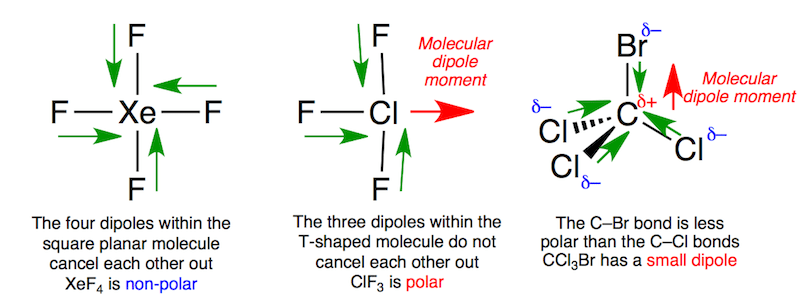
Determine the central atom in this molecule. We ve used 6 valence electrons. We re being asked to determine the lone pairs that surround the central atom in the lewis structure of clf 3.
That means we have 4 valence electrons left over.
And then around the outside we ve used 6 8 and 24. 7 x 3 7 28 bind all three f to cl at least with one bond. Alternatively a dot method can be used to draw the clf 3 lewis structure. A video explanation of how to draw the lewis dot structure for chlorine trifluoride along with information about the compound including formal charges pola.
