Cif3 Expanded Octet Lewis Structure. In drawing the lewis structure for pcl 5 there is a total of 40 valence electrons to put in 5 5x7 40. Lewis dot structures are useful to predict the geometry of a molecule.
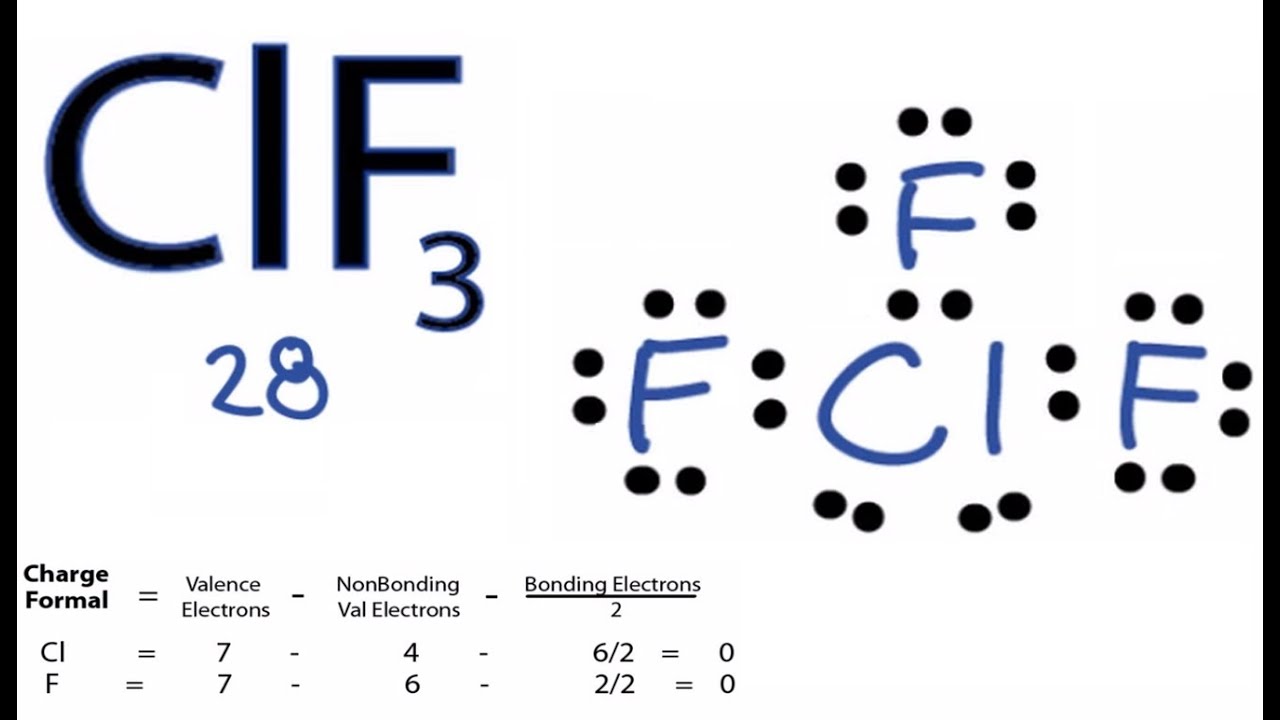
Most lewis structures will follow the octet rule which states that the outer valence shell is stable when it has eight electrons. In addition bf 3 will react with ammonia nh 3 to form a stable compound nh 3 bf 3 for which a lewis structure can be drawn that shows boron with a complete octet. For more complicated molecules and molecular ions it is helpful to follow the step by step procedure outlined here.
Boron trifluoride ammonia complex.
You should find that accounts for all the ve that there are too many and has an expanded octet electrons to only have 8 around around only the central atom. There are many exceptions to this rule but it should be used as a general guide for creating lewis structures. This covalent compound nh 3 bf 3 shows that boron can have an octet of electrons in its valence level. Writing lewis structures with the octet rule.
