C2h4 Lewis Structure Resonance. In a double bond two pairs of valence electrons are shared for a total of four valence electrons. Include any resonance structures.
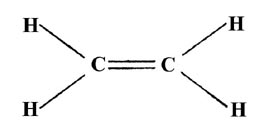
Drawing the lewis structure for c 2 h 4 named ethene requires the use of a double bond. Draw the lewis structure for the ethylene c2h4 molecule. Be sure to include all resonance structures that satisfy the octet rule.
If you remove the single non bonded electron from carbon you will form a carbo cation.
Draw the lewis structure for the ethylene c2h4 molecule. Include any resonance structures. The number of valence electrons of c2h4 is 12 but usually it is 2 charge so in that case it will be 10. Put one electron pair in each bond4.
