Br2 Covalent Bond Lewis Structure. 0 nonpolar covalent. Covalent bonds consist of pairs of electrons shared by two atoms.
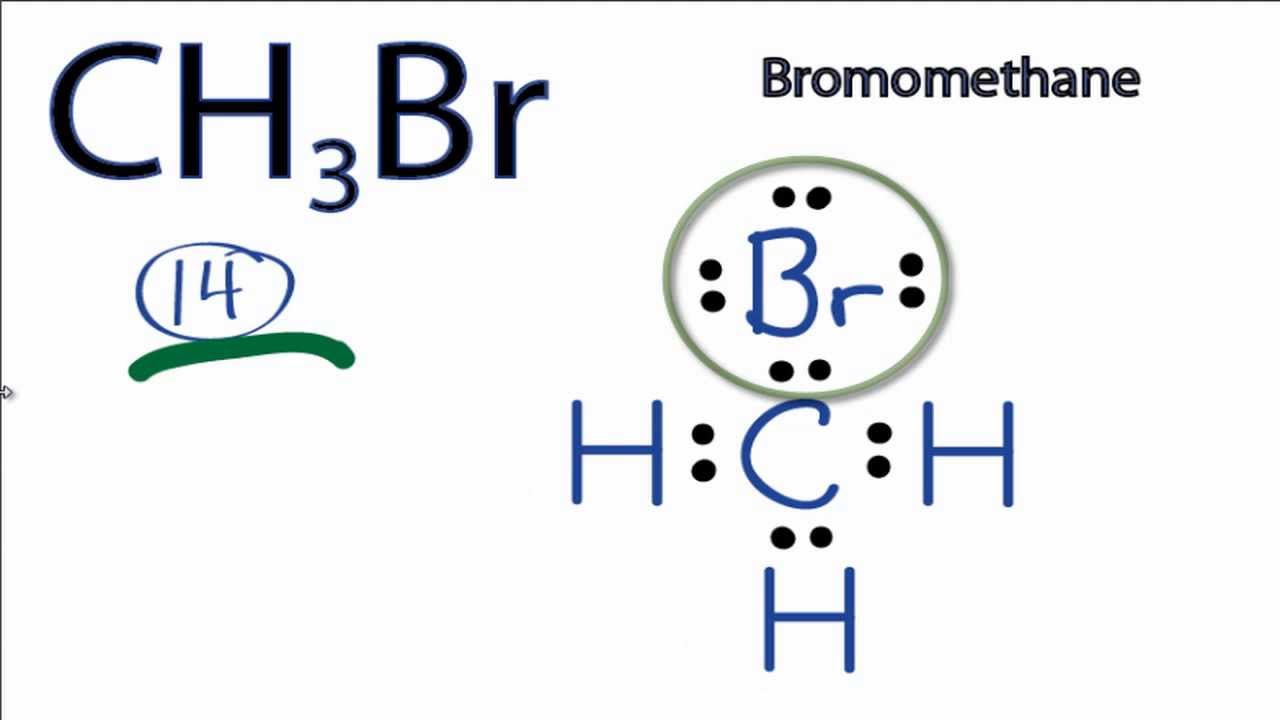
They differ in their structure and properties. A diatomic molecule with a triple covalent bond is. The br2 is a diatomic molecule that has a total of 14 valence electrons.
Start studying covalent naming and lewis structures.
In the br2 molecule the lewis structure of br2 is similar to that of f2 cl2 etc. A plot of the overall energy of a covalent bond as a function of internuclear distance is identical to a plot of an ionic pair because both result from attractive and repulsive forces between charged entities. A step by step explanation of how to draw the br2 lewis structure dibromine. The percentage ionic character and the type of bond in br2 electronegativity for br is 2 8 is a.
