Ash3 Lewis Structure. I cant draw the structure by this. Remember that hydrogen h atoms always go on the outside of a lewis structure.
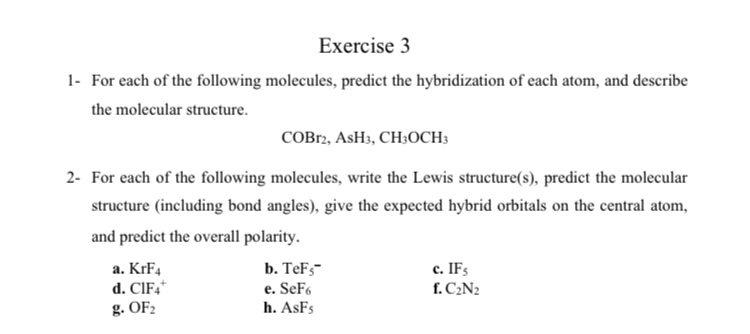
As 5a 1 5 e 5 e. This is the ash3 lewis structure. I would draw ash3 as a tetrahedral because of the 3 bonds lone pair but i thought when naming you disregard the lone pair so it would be a trigonal planar.
As 5a 1 5 e 5 e.
I would draw ash3 as a tetrahedral because of the 3 bonds lone pair but i thought when naming you disregard the lone pair so it would be a trigonal planar. A step by step explanation of how to draw the ash3 lewis structure for arsenic trihydride for the ash3 lewis structure calculate the total number of valence. The arsenic atom goes in the center of the lewis structure since it is the least electronegative atom. It forms the compound ash3 draw the shape of an ash3 molecule including any lone pairs of electrons.
